The erythropoietin receptor expressed in skeletal muscle is essential for mitochondrial biogenesis and physiological exercise
A study by Kirsten Nijholt and Daan Westenbrink was recently published in Pflügers Archiv-European Journal of Physiology. They studied the role of the erythropoietin receptor in cardiac and skeletal muscle and its relevance for physiological exercise. Other team members included Laura Meems, Willem-Peter Ruifrok, Alexander Maass, Salva Yurista, Mario Pavez-Giani, Belend Mahmoud, Anouk Wolters, Dirk Jan van Veldhuisen, Wiek van Gilst, Herman Silljé and Rudolf de Boer.
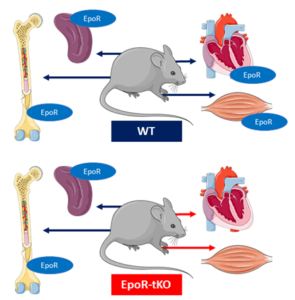
Current literature suggests that the erythropoietin receptor (EpoR) is present beyond the hematopoietic system, including cardiac and skeletal muscle tissue. Studies with pathological stressors have provided the evidence that the EpoR has protective effects. Yet, its role for normal muscle physiology remained unknown.
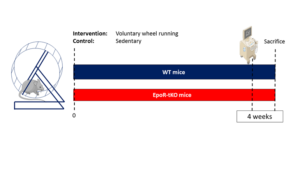
The team performed experiments with a mouse model in which there was tissue specific knock out of the EpoR (EpoR-tKO mice), with preserved hematopoiesis. First, baseline phenotyping of cardiac and skeletal muscle was performed, including mitochondrial phenotyping. Next, mice were subjected to four weeks of voluntary wheel running. The authors demonstrated that EpoR deficiency in skeletal muscle resulted in impaired mitochondrial biogenesis. In addition they observed that ablation of the EpoR in both cardiac and skeletal muscle decreased exercise performance. Finally, skeletal and cardiac muscle growth and angiogenesis adaptations in response to exercise were blunted in EpoR-tKO mice.
The authors concluded that the EPO-EpoR system regulates mitochondrial biogenesis in skeletal muscle. Additionally, impaired mitochondrial biogenesis in EpoR-tKO mice was associated with reduced exercise performance, suggesting that the extra-hematopoietic EpoR is critical for exercise-induced adaptation. Therefore, the EpoR in skeletal muscle could be a potential novel target to improve skeletal muscle quality. Hence, these findings can be of translational relevance for patients with muscle fatigue, mitochondrial myopathies or heart failure-associated exercise intolerance.