
Pregnancy-related heart failure
Peripartum cardiomyopathy (PPCM) is a rare but potentially fatal cardiac complication of pregnancy. The disease is characterized by heart failure in women presenting in the last trimester or in the first 5 months following childbirth. It affects between 1:1000-3000 pregnancies in Europe to 1:300 pregnancies in geographical hotspots like Haiti. Heart failure may spontaneously resolve in half of the PPCM patients; other patients may have persisting heart failure while up to 28% of patients die. PPCM is a complex disease and may be misdiagnosed due to clinical similarities with other pregnancy-related diseases (e.g., preeclampsia). Remarkably, PPCM can occur in women with an otherwise normal pregnancy. The cause remains unknown, but various risk factors have been proposed including age, ethnicity, nutrition, and number of previous pregnancies.
The most frequently cited hypothesis for PPCM pathogenesis is based on two independent mouse models that harbor a conditional cardiac knockout of either STAT3 (Hilfiker et al. Cell 2007) or PGC-1α (Patten et al. Nature 2012). Cardiac-specific knockout of these genes in mouse hearts led to increased oxidative stress, which stimulated the secretion of the lysosomal peptidase cathepsin D that in turn cleaved the circulating nursing hormone prolactin. The resulting 16-kDa prolactin fragment promotes apoptosis in endothelial cells. These mouse models revealed significant vascular damage in late pregnancy and consequent heart failure. Additional studies showed that 16-kDa prolactin induced miR-146a from affected endothelial cells to adjacent cardiomyocytes, resulting in cardiomyocyte apoptosis.
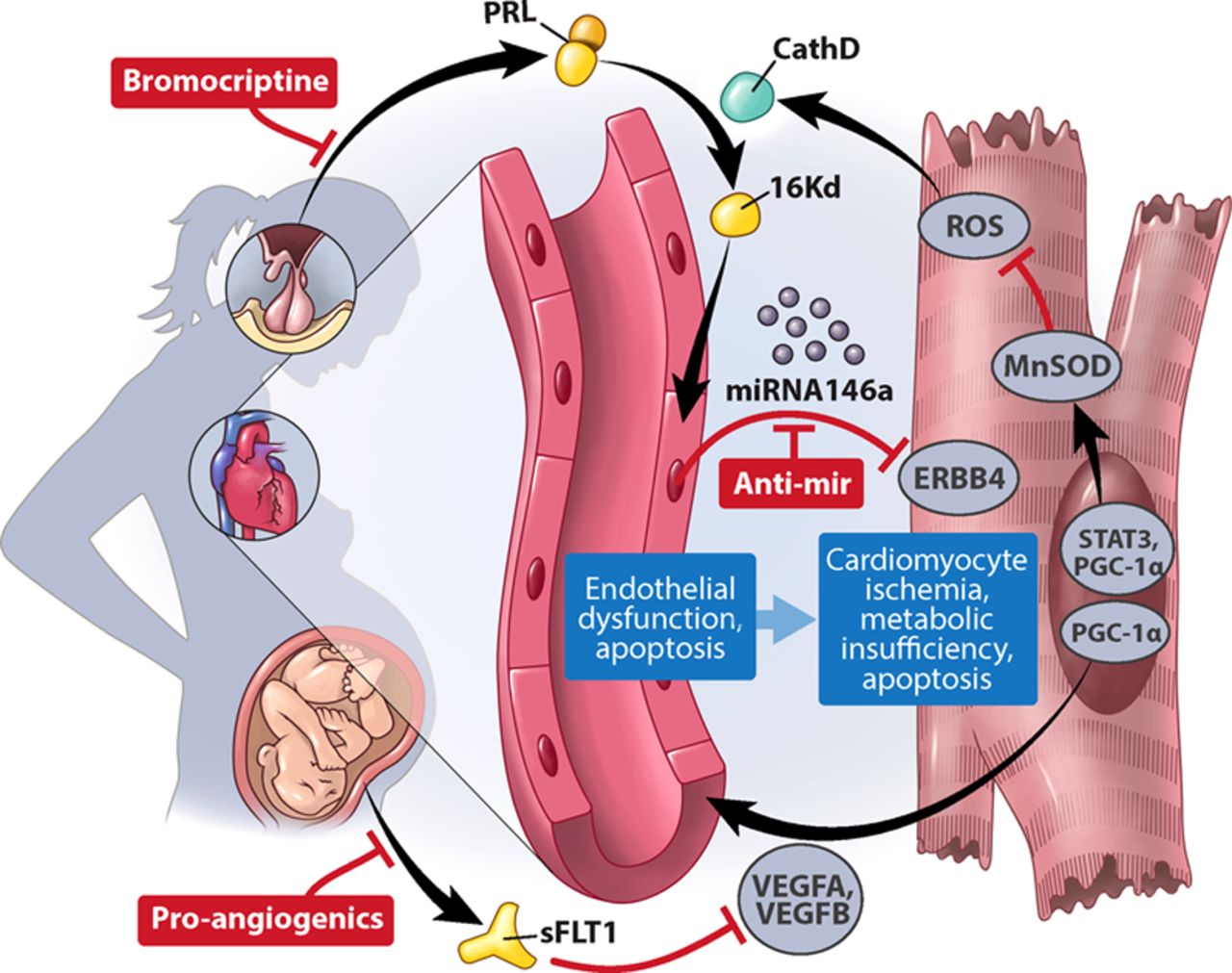
Proposed PPCM pathogenesis (from Arany and Elkayam, Circulation 2016).
Martijn Hoes set out to study the molecular mechanisms underlying the pathogenesis of this disease and demonstrated that cathepsin D may be secreted by the stressed heart in general, but that this may not necessarily lead to heart failure in the absence of prolactin (Eur J Heart Fail, 2020). Moreover, he also indicated that circulating cathepsin D levels also reflected heart failure severity in patients included in the BIOSTAT-CHF study. As part of a collaboration with the group of Denise Hilfiker-Kleiner, Martijn demonstrated that the 16 kDa prolactin fragment could only elicit a detrimental effect in the presence of plasminogen activator inhibitor-1 (PAI-1), which was previously unknown (Cardiovascular Research, 2020). In the search for the actual cause of the disease, Martijn generated induced pluripotent stem cell (iPSC) lines from multiple PPCM patients and healthy relatives. These iPSCs were differentiated toward cardiomyocytes that allowed him to study molecular pathways in PPCM patient-derived cardiomyocytes compared that could be offset to respective healthy cardiomyocytes. Through this comparison, it became clear that PPCM patient-derived cardiomyocytes could not effectively switch between different metabolic pathways when challenged (Circulation 2020).
Martijn completed his PhD under supervision of Peter van der Meer as part of the STOP-HF project. His main topics pertained to in vitro disease modeling, iron deficiency, and PPCM, which resulted in several publications as a result of successful collaborations within the department and with national and international groups.
Martijn currently focuses on maternal cardiac metabolism during pregnancy.
This post concludes the eight-part series about our projects related to sex differences in heart disease.