Inclusion GIPS-IV trial completed
The Groningen Intervention study for Preservation of cardiac function with Sodium thiosulfate in ST-elevation myocardial infarction (GIPS-IV)
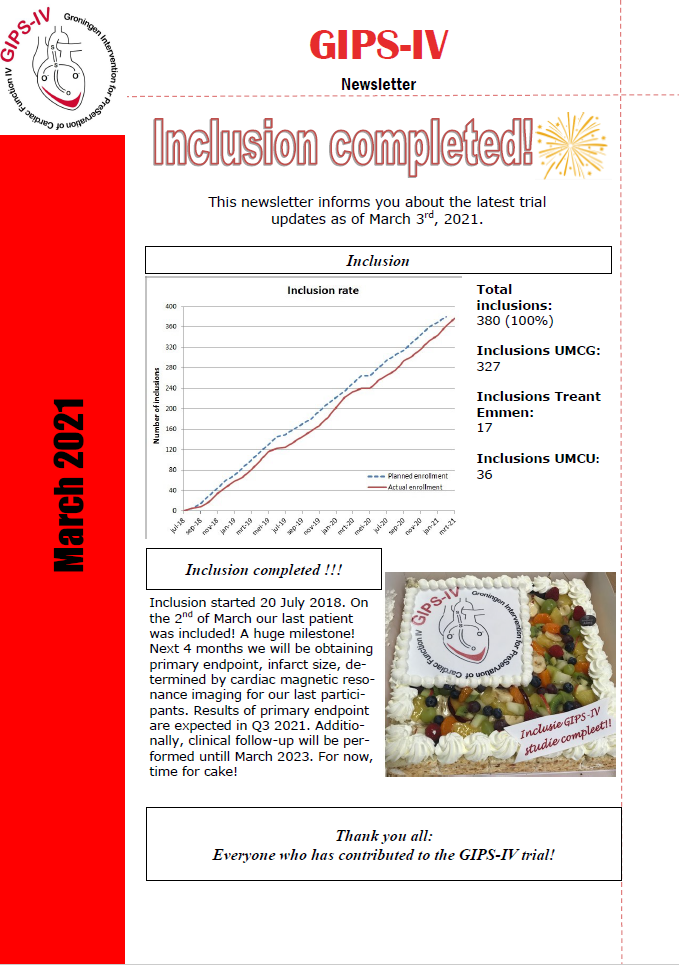
In March 2021 the inclusion of the GIPS-IV trial was completed, a huge milestone! In 2.5 years, 380 participants were enrolled across 3 centers: University Medical Center Groningen, University Medical Center Utrecht and Treant hospital Emmen. Due to the huge effort of all recruiting centers, we experienced only very little delay from COVID-19. See also our latest newsletter below.
We kindly thank all our participants and the study teams.
GIPS-IV trial
The Groningen Intervention study for Preservation of cardiac function with Sodium thiosulfate in ST-elevation myocardial infarction (GIPS-IV) is a randomized placebo-controlled, multicenter trial. In this study, sodium thiosulfate or matching placebo was administered twice during admission for STEMI, on top of standard treatment. After discharge, patients will be followed-up by telephone for 2 years, which will last until March 2023. Four months after STEMI we will obtain primary endpoint, infarct size, as measured by CMR. Additionally, during this visit also echocardiography and electrocardiography and laboratory sampling are performed. In the summer of 2021 we expect the data collection for primary endpoint to be completed.
We expect our results later this fall and will be happy to share our first findings.
Read more about:
Ischemic Heart disease research team