Completed enrollment of the PUSH AHF trial!
Our team of PUSH AHF trial is incredibly excited and proud to report that the enrollment of the Pragmatic Urinary Sodium AlgoritHm in Acute Heart Failure (PUSH AHF) Study is complete. This could not have done this without the patients, nurses and physicians of the Cardiology Department of the University Medical Center Groningen (UMCG).
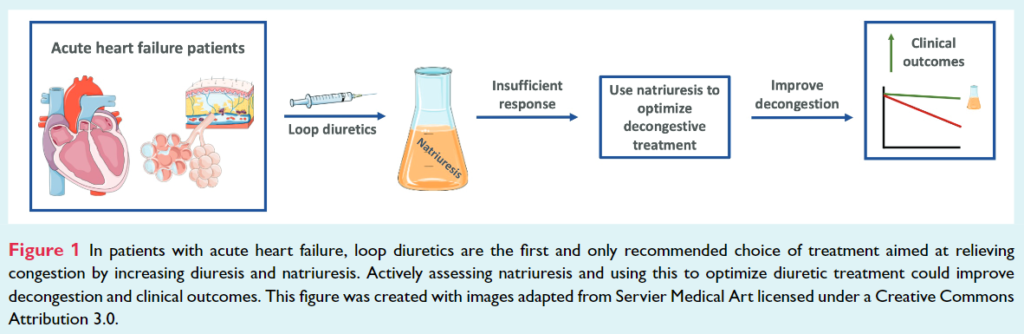
Recent studies have shown that measuring natriuresis early after hospital admission could reliably identify patients with a poor diuretic response during hospitalization who might require enhanced diuretic treatment. Insufficient diuretic response frequently occurs in patients admitted for acute HF and is associated with worse clinical outcomes.
The PUSH AHF trial is a pragmatic, single-center, randomized, controlled, open-label study, and aimed to recruit 310 acute HF patients requiring treatment with intravenous loop diuretics (Table 1). This study will test the hypothesis that natriuresis-guided therapy, using a pre-specified stepwise diuretic treatment approach, in patients with acute HF improves natriuresis and clinical outcomes (Figure 1).
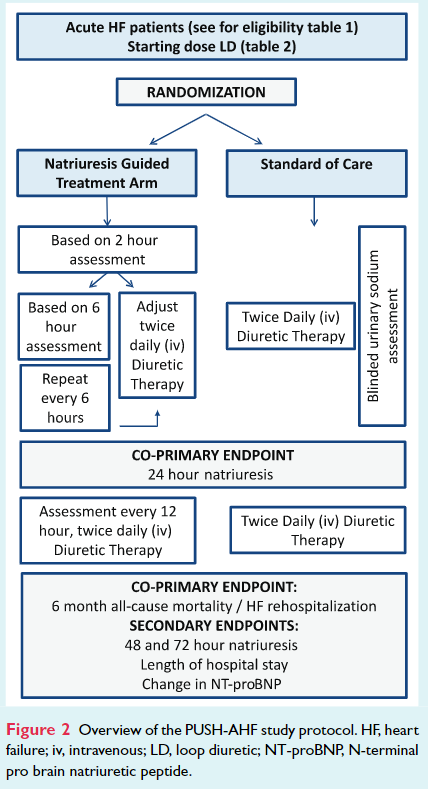
Patients were randomized to natriuresis-guided therapy or standard of care (Figure 2). The co-primary endpoint is 24-h urinary sodium excretion after start of loop diuretic therapy and a combined endpoint of all-cause mortality or first HF rehospitalization at 6 months. Secondary endpoints include 48- and 72-h sodium excretion, length of hospital stay, and percentage change in N-terminal pro brain natriuretic peptide at 48 and 72 h.
The results are expected in the summer – stay tuned!
To read the full published study protocol, please click here.